
The US Food and Drug Administration (FDA) approved the groundbreaking CRISPR gene-editing technology on Friday (8 Dec), heralding a new era in the fight against genetic diseases.
Vertex Pharmaceuticals and CRISPR Therapeutics, the pioneering companies behind the innovation, are now poised to introduce the world’s first CRISPR therapy, a Nobel-prize-winning technology.
The approved therapy, named Casgevy, is now approved to treat sickle cell patients. By editing the DNA to correct flawed red blood cells, the treatment offers the prospect of a disease-free life.
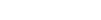
Access deeper industry intelligence
Experience unmatched clarity with a single platform that combines unique data, AI, and human expertise.
However, experts emphasise that the current application of CRISPR by these companies involves a less risky approach. Instead of editing the disease-causing mutation directly within the body, the therapy employs an “ex-vivo” technique—extracting blood stem cells, editing them in a controlled laboratory environment, and subsequently reintroducing them into the patient’s body.
This method ensures a safer procedure, as scientists can scrutinise and confirm the accuracy of the DNA edits before the cells are transplanted.
While this CRISPR therapy represents a significant breakthrough for sickle cell disease, the true potential of CRISPR lies in its ability to edit genes “in vivo,” or within the body, without the need for laboratory manipulation.
This advancement could simplify and streamline treatments for diseases affecting various organs, such as the brain, heart, and lungs, making them less strenuous for patients.
Despite the promise of the newly approved therapy, experts caution that it involves challenging and risky procedures, including chemotherapy to facilitate the removal of cells from the bone marrow. These extracted cells are then replaced with the edited ones, which can be a painful and strenuous process for patients.
CRISPR technology, renowned for its ability to cut and paste DNA with unprecedented precision, has garnered attention for its potential to address genetic diseases at the molecular level.
Early targets, such as sickle cell disease, have proven amenable to CRISPR due to the simplicity of the genetic mutation causing the ailment. Recent innovations, including prime editing, have further enhanced the precision and efficiency of gene editing.
US President Joe Biden lauded the FDA’s approval, underscoring the significant medical advancement that holds promise for developing life-saving treatments and providing hope for millions affected by rare diseases.
US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHowever, concerns linger about the accessibility and safety of CRISPR therapies. Vertex has disclosed a treatment cost of $2.2m per patient, raising questions about affordability for individuals, particularly those reliant on Medicaid.
The FDA has mandated a 15-year monitoring period for patients undergoing CRISPR treatments to assess long-term safety and its potential unforeseen impacts.







