Researchers have shown how it is possible to create a lithium-ion battery that can charge in minutes but still operate at a high capacity.
The discovery could improve battery performance across an array of technologies, such as electric vehicles, portable electronics and solar grids.
Scientists at the Rensselaer Polytechnic Institute, New York, did so by improving materials used for the electrodes, parts of the battery that facilitate charging and discharging.
They found that substituting part of the battery for a different material, vanadium disulfide (VS2), successfully improved its efficiency.
“It gives you higher energy density, because it’s light. And it gives you faster-charging capability because it’s highly conductive. From those points of view, we were attracted to this material,” said Nikhil Koratkar, professor of mechanical, aerospace, and nuclear engineering at Rensselaer, and corresponding author of the paper.
In standard lithium-ion batteries, lithium ions move between two electrodes – the anode and cathode.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThe anode is usually made from graphite, while the cathode from lithium cobalt oxide. Researchers switched to the lithium cobalt oxide cathode to vanadium disulfide, and found that it improved battery performance.
VS2-TiS2 key to lithium-ion battery improvement
Scientists have known about the benefits offered by VS2 for some time, but have struggled to overcome the material’s instability, which leads to a shorter battery life.
The study, published in the scientific journal Nature Communications, shows how the Rensselaer researchers identified the cause of the instability problem and found a way to solve it.
They discovered that inserting lithium triggered a distortion that broke up the VS2 flakes and caused instability in the battery.
To prevent this from happening, the team covered VS2 flakes with a nanolayered coating of titanium disulfide (TiS2). This stabilised the VS2 flakes and therefore improved the battery’s performance.
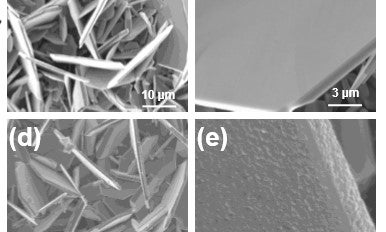
“This was new. People hadn’t realised this was the underlying cause,” said Koratkar, who is also a professor in the Department of Materials Science and Engineering. “The TiS2 coating acts as a buffer layer. It holds the VS2 material together, providing mechanical support.”
The results were electrodes that could still store a lot of charge for their size, which means batteries could be more compact.
Because the VS2-TiS2 combination is electrically conductive, the electrodes can maintain a reasonable capacity during faster charging.
Read more: How Arsenal’s battery system can power the Emirates for 90 minutes