Cannabis has been receiving some serious media attention in 2018, and a rise in medical cannabis research is following.
Canada became the second country in the world to legalise it for recreational use, after Uruguay. From a medical standpoint, the first patient in the UK finally succeeded in obtaining a long-term personal licence for medical cannabis, a move that could pave the way for reforms on its medical use.
In the US, the FDA approved the first pure cannabis-derived drug, Epidiolex (cannabidiol, CBD), a liquid formulation developed by GW Pharmaceuticals for the treatment of two rare and severe forms of epilepsy: Lennox-Gastaut syndrome and Dravet syndrome.
Medical cannabis research and the Epidiolex milestone
Naturally occurring cannabis contains a group of compounds known as cannabinoids. To date, only two cannabinoids have been well characterised, CBD and THC. The former is the active ingredient in Epidiolex and has anticonvulsant properties, while the latter is the psychoactive compound that can cause the “high”.
The approval of Epidiolex is certainly a major milestone, especially for the epilepsy community; however it is worth noting that CBD is still a Schedule I substance because it is a chemical component of the cannabis plant, meaning that the drug cannot reach the market until the Drug Enforcement Administration (DEA) changes how it classifies this compound.
It is expected that CBD will be downgraded to a Schedule II or III drug, with the DEA’s decision being announced by the end of September at the latest.
US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe final decision will be crucial for Epidiolex’s launch, although it does not mean that that cannabis itself will be downgraded too; not for the time being, at least. However, the downgrading of CBD is likely to stimulate more research into cannabis-based products.
The future of medical cannabis research
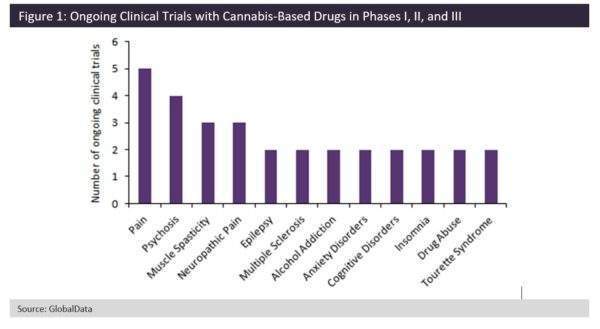
According to GlobalData, there are currently 60 ongoing clinical trials with cannabis-based products.
The majority of these products are being tested in pain or psychosis, although there is a wide variety of indications where cannabis-based products could potentially be used.
The fact that cannabis has been classed as Schedule I, the highest classification possible, has certainly posed a hurdle in R&D, but with the support of scientific data we have been seeing in recent years and the upcoming downgrade of CBD specifically, we can expect a boom for medical marijuana in the near future.